Infecting people on purpose:
The power of human challenge studies in tackling infectious disease
Could deliberately infecting healthy volunteers with a disease help to save the lives of many more people? While this approach seemingly contravenes the guiding principle of medical ethics to "first, do no harm", the practice of intentional infection for health benefit has a long history, and has contributed to major medical advancements over the centuries.
Human challenge studies can be traced back to the world’s first vaccine - the smallpox vaccine - when Edward Jenner deliberately inoculated a young boy with cowpox and then repeatedly exposed him to smallpox to create immunity.
Fortunately, since then, significant strides have been made in improving the ethical and regulatory standards for this type of research. Such studies are now designed to minimise risk to research participants, and the health and safety of volunteers is paramount.
In recent times, human challenge studies have been indispensable in the fight against some of the world’s greatest killers, supporting the development of vaccines for malaria, typhoid, cholera, and tuberculosis.
But how?
Often smaller, shorter, and less expensive than traditional clinical trials, human challenge studies can expedite the typically lengthy and costly process of vaccine development. They can give researchers unique insights that other studies cannot because, unlike natural infection, they are tightly controlled. By intentionally exposing healthy volunteers to infectious pathogens in a carefully managed environment, scientists can follow the course of infection in minute detail and assess the efficacy of potential vaccines and therapies.
Imperial College London is a global leader in such studies, pioneering the development of human challenge models for a wide range of pathogens - from COVID-19 to malaria. We spoke to some of our researchers using the approach to advance our understanding of these diseases and help develop next-generation vaccines.
Of men, not mice: Advancing respiratory research
Professor Peter Openshaw

After spending many years using mouse models to investigate lung immunology and inflammation, Professor Peter Openshaw realised that they could not fully capture key features of human disease.
"While they laid a great foundation, I eventually came to the conclusion that we had to move into human studies if we’re going to really understand what's going on in man," acknowledges the Professor of Experimental Medicine at Imperial’s National Heart and Lung Institute (NHLI).
Sure enough, Professor Openshaw has now been running human challenge studies for over 15 years, supervising and leading the establishment of challenge models for respiratory viruses such as influenza, RSV and most recently, COVID-19.
He credits the invaluable contributions of research volunteers and the support of the public in getting them off the ground.
"This has helped us enormously to do some quite difficult studies in recent times," he says.
"For me, the participants are very much part of the research team, and I think it's important that they’re treated in that way."
"Many of our volunteers are interested in the science. They become fascinated with what we're discovering, and they really want to know what the outcomes are. So, for me, the participants are very much part of the research team, and I think it's important that they’re treated in that way."
Now, as co-Director of the HIC-Vac network, Professor Openshaw is dedicated to advancing the use of human challenge studies to help develop future vaccines, especially in low- and middle-income countries (LMICs) where high-impact pathogens cause the greatest morbidity and mortality.
"We need to know whether the studies we’re doing in rich parts of the world are relevant to vaccine development for developing countries; it's in resource-poor areas with high rates of infectious diseases that vaccines can potentially have the greatest impact," he explains.
Comprising an international network of researchers, HIC-Vac aims to grow and develop human challenge studies in the UK and LMICs by enabling the open sharing of knowledge and expertise.
"It's in resource-poor areas with high rates of infectious diseases that vaccines can potentially have the greatest impact."
Given the nature of the research that the network facilitates, effective knowledge-transfer and capacity building is crucial for developing best practice guidance, explains Professor Openshaw.
"One of our first projects supported the process of setting up human challenge studies in Zambia. Through engagement with local authorities, we facilitated the development of ethical and regulatory frameworks to guide the conduct of these studies. This was the first time that officials in Zambia engaged with human challenge studies, and this has since led to the development of a local framework with support from the Wellcome Trust."
Following recent renewed funding for HIC-Vac, he describes what the coming years hold for the network.
"We will extend our reach into parts of the world that previously haven't attempted to do human challenge studies and link them with groups already doing these types of studies so that they can receive the advice and support of those with experience.
"These investments take time to bear fruit, but HIC-Vac is enabling progress to be made in areas that were previously static."

Rising to the challenge: Facing COVID-19
Professor Chris Chiu

"What has really interested me throughout my career is why, when some people are exposed to a virus, they get really sick and end up in hospital, but other people exposed to the same virus may have no symptoms at all," says the Professor of Infectious Diseases, Chris Chiu.
"My research tries to understand that and harness human challenge studies to make better vaccines and interventions."
Professor Chiu heads up a world-leading research group that uses human challenge studies to investigate respiratory viruses like RSV, influenza and most recently, SARS-CoV-2 (the virus which causes COVID-19).
In February 2021, Professor Chiu led the world’s first COVID-19 challenge trial in which healthy young adults were deliberately infected with the virus. The study was the first in the world to capture detailed data on the full course of COVID-19 infection, even before symptoms appeared.
"My research tries to harness human challenge studies to make better vaccines and interventions."
Among one of the most important early discoveries the team made was that lateral flow tests (LFTs) were a reassuringly reliable indicator of when someone was unlikely to infect others and could therefore come out of isolation.
"We showed how LFTs could be used to reliably test people towards the tail end of their infection and reassure people that they were no longer infectious and safe to go out," he explains.
"It therefore provided the information we needed to understand how to get out of lockdown, quarantine, self-isolation, and so on.
"No other research at the time was able to do that and it gave not only the UK, but also the whole world the confidence to use LFTs as a way to rationally release people from self-isolation with minimal risk to the community."
"It provided the information we needed to understand how to get out of lockdown, quarantine, and self-isolation."
Recent analysis of samples obtained from the study also uncovered why some people can fend off COVID-19. The findings suggest that these individuals have unique immune responses in the lining of their nose that enable them to identify the virus and very quickly stop it from gaining a foothold to cause infection.
The study has continued to lay the groundwork for using the human challenge model to investigate further aspects of COVID-19.
Now, as principal investigator for the Mucosal Immunity in human Coronavirus Challenge (MusiCC) project, Professor Chiu and an international consortium of researchers specialising in human challenge studies aim to develop next-generation vaccines that not only reduce disease severity, but also stop people from catching COVID-19 and other coronaviruses in the first place.
"It became very clear during the pandemic that the vaccines we have are very good at preventing severe disease, but not good at preventing the spread of the infection. In other words, the pandemic continued despite the fact that many people were vaccinated," he says.
"So, the aim of MusiCC is to develop ways to accelerate the delivery of vaccines which are able to block or reduce transmission, as well as block infection and disease."
Unlike traditional vaccines which are injected into muscle, MusiCC will test the potential of nasal spray or inhaled vaccines against beta coronaviruses – the pathogen family that includes SARS-Cov-2, Middle East respiratory syndrome coronavirus (MERS-CoV) and seasonal common cold viruses.
Professor Chiu believes strongly in the benefit his team’s research will lead to for patients.
"Our volunteers and staff invest a huge amount of their time and effort into these studies, so it's important that we get as much scientific and public health value out of them as possible. That's really at the forefront of everything we do."


30 years and counting: Breaking malaria's grip
Professor Faith Osier

While malaria has been eradicated in several countries around the world, the mosquito-borne disease continues to be a pervasive threat in sub-Saharan Africa, claiming the lives of over half a million people each year. Most are children under five years of age.
"The continent is being held back by a disease that can be treated and prevented. To do nothing is criminal," says Professor Faith Osier, Co-Director of Imperial’s Institute of Infection and Chair of Immunology and Vaccinology in the Faculty of Natural Sciences.
A passionate advocate for Africa, its science and scientists, Professor Osier is on a mission to make malaria history through vaccination.
"I’ve been working on malaria for around 30 years, trying to understand how people become immune", she says.
"The continent is being held back by a disease that can be treated and prevented. To do nothing is criminal."
Brought up in Nairobi, Kenya, Professor Osier first witnessed the devastating effects of malaria when working in paediatrics as a junior doctor in a rural district hospital.
"I was seeing firsthand the destructive impact it was having in my country."
It was here where Professor Osier’s drive to understand the mechanisms of immunity against malaria was ignited.
"The children that I was treating on the ward every day were getting the disease very badly and developing severe complications afterwards, or sometimes even losing their lives. In contrast, their parents who were bringing them to hospital – who lived in the same house and were getting bitten by the same mosquitoes – were not getting ill", she explains.
"It became obvious that their immune systems had somehow learnt to counter the infection. That’s how I became interested in understanding immunity because if you can understand that, then you can design a vaccine that can do the same thing but not take as long as naturally acquired immunity."
A human red blood cell infected with the malaria parasite
A human red blood cell infected with the malaria parasite
In an effort to design better vaccines, a team from the KEMRI-Wellcome Trust Research Program in Kilifi, Kenya, where Professor Osier previously worked, led the Controlled Human Malaria Infection in Semi-Immune Kenyan Adults (CHMI-SIKA) study. It involved purposely infecting semi-immune adult volunteers (those who had previously been naturally infected multiple times) from Kenya with malaria.
"What's been absolutely magical about this human challenge study is that we’ve been able to show that this concept of ‘immunity in the community’ is really true", she says.
"We saw from our experiment that when you infect volunteers who’ve had malaria many times before, their immune systems simply gobble it up and it doesn’t touch them at all. They don’t get sick.
"But the next step was to unravel what was going on at a molecular level in order to inform the design of better vaccines."
The study revealed that the principle behind most vaccines – generating antibodies that bind to the infectious pathogen and fight it off – is only part of the story for malaria infection. Instead, antibodies that effectively ‘recruit’ other parts of the immune system were found to provide enhanced protection against malaria infection.
As a result of their findings, the international research team has identified a possible way to produce these antibodies and is now creating experimental vaccines on various platforms to find the most effective one.
"Every life has value and the potential to achieve something great. Unfortunately, a lot of that potential is wasted because of this disease."
Reflecting on her own experiences of malaria and what motivates her work, Professor Osier states: “As a mother, when you’re repeatedly faced with parents who’ve lost a child and have experienced that kind of devastation, you begin to understand what a huge impact it has on human life.
"I believe that every life has value and the potential to achieve something great. Unfortunately, a lot of that potential is wasted because so many people succumb to this disease.
"So, when we intervene and prevent disease, we do good for humanity."


Low profile, high risk: Unmasking the deadly threat of invasive
non-typhoidal Salmonella
Dr Malick Gibani

"Salmonellosis is a disease with an 'image problem'," states Dr Malick Gibani, Clinical Lecturer in the Department of Infectious Disease at Imperial.
"Somewhere in the region of 15 to 30 per cent of children infected with invasive non-typhoidal Salmonella die, and there aren't many illnesses like this where around a third of people die."
Despite being one of the leading causes of bloodstream infections in sub-Saharan Africa, invasive non-typhoidal Salmonella (iNTS) is poorly understood. But Dr Gibani hopes to change that.
"Malaria, COVID-19, and influenza are all illnesses that have a high profile. They don’t take a huge amount of explaining to healthy volunteers. But when it comes to NTS, it’s not typically something that people have heard about," he says.
"From an advocacy perspective, this is something that we’ve spent quite a lot of time thinking about because we’re trying to convince decision-makers and stakeholders that iNTS is something that we critically need to invest in."
"There aren't many illnesses like this where around a third of people die."
NTS is the name given to a group of bacteria that typically cause foodborne illness. Most people experience diarrhoea and fever and recover over the course of a few days. These infections are usually linked to contaminated food or water and differ from typhoid fever, which is caused by another, specific type of salmonella.
In some instances, these bacteria cross the gut and enter the bloodstream to cause a much more severe form of the disease – called invasive disease. This is almost always seen in medically vulnerable populations (infants, the elderly and immune-compromised patients), where iNTS can be life-threatening and can result in serious complications such as septicaemia and kidney failure.
"To a certain extent, this is a geographically restricted disease where we see the highest burden of the disease in sub-Saharan Africa," Dr Gibani explains.
"It’s predominantly seen in young children who are malnourished or immuno-compromised. There is a particularly strong association with other infections like malaria and in some cases, advanced HIV infection.
"It’s definitely a neglected disease but I think it's something where we can find an effective solution for, like a vaccine."
That’s why, as part of the Challenge Non-Typhoidal Salmonella (CHANTS) study, Dr Gibani and his team are working with Imperial College Healthcare NHS Trust to run and develop a human challenge model that can be used to test and accelerate vaccines for NTS – a world first.
Known as a ‘dose-escalation’ study, CHANTS aims to determine the lowest possible dose of bacteria needed for volunteers to meet the criteria for diagnosis. Once the optimal dose is established and the model is proven safe, it can be replicated in future to test different vaccines.
"The fact that this study is even happening at all is a major achievement. There’s already a lot of interest in the data that we're generating, both from key stakeholders in the regulatory space and vaccine stakeholders," he says.
Having taken place across 2024, Dr Gibani is keen to translate the research findings into real-world practice and decision-making.
"We want to build partnerships with vaccine developers so that we can use the model to accelerate potential candidates and get vaccines into the arms of people who need it the most."
Indeed, one key partner that Dr Gibani’s group is working closely with is GSK Vaccines Institute for Global Health (GVGH) to study antibody responses to Salmonella. They develop and research vaccines aimed at preventing and treating infectious diseases that disproportionately affect people in LMICs, with a particular focus on diseases with antimicrobial resistance (AMR) potential.
"Waiting any longer to tackle this global health threat is not an option."
“Salmonella has been included as a priority pathogen in the World Health Organization’s updated Bacterial Priority Pathogens List – a list of drug-resistant bacteria most threatening to human health,” he explains.
“Waiting any longer to tackle this global health threat is therefore not an option. iNTS demands the research and development community’s immediate attention.”
Dr Gibani and his team hope to develop further challenge models and identify ‘correlates of protection’ for iNTS. These are specific measurable signs in the body, like antibodies or immune responses, that indicate someone is protected from a disease. Researchers use these markers in vaccine development to understand if a vaccine is effective in providing immunity without waiting for actual infections to occur.
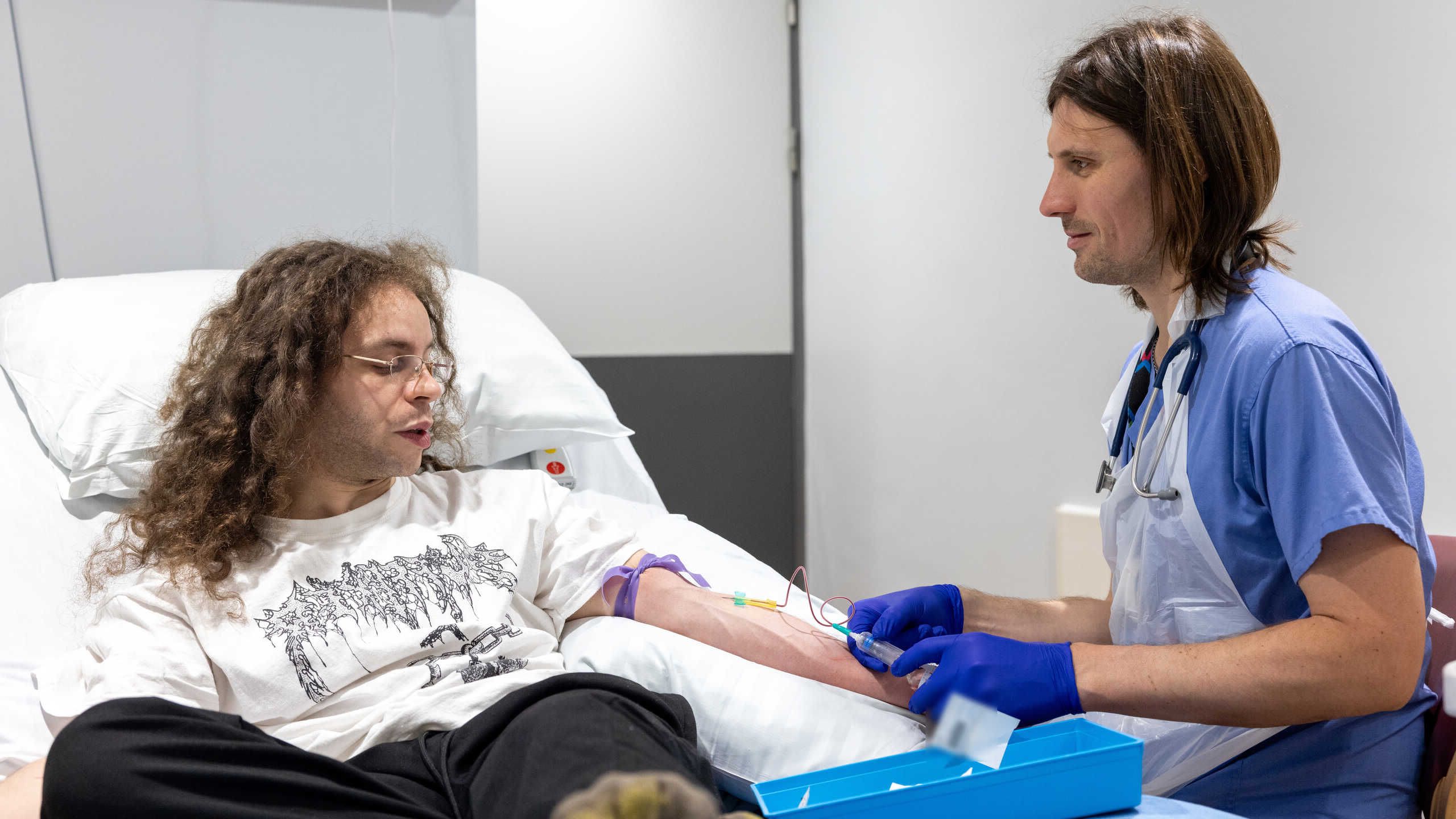

Something in the air: Unravelling COVID-19 transmission
Professor Wendy Barclay

As someone who is fascinated by the transmission routes of respiratory viruses like influenza, it’s perhaps unsurprising that Professor Wendy Barclay has spearheaded efforts to apply this knowledge to COVID-19.
"What does it take for a virus to jump across from animals into humans? And then what does it take once it's in a human to become airborne? That’s what I’m really interested in," says Professor Barclay, Head of Imperial’s Department of Infectious Disease.
During the height of the COVID-19 pandemic, she worked extensively with Imperial colleagues like Professor Chris Chiu, Dr Anika Singanayam and Dr Jie Zhou to provide granular insights into how people infected with COVID-19 spread the virus to their immediate surroundings.
Dr Jie Zhou, Research Associate in The Barclay Lab
Dr Jie Zhou, Research Associate in The Barclay Lab
Harnessing her expertise in molecular virology, she facilitated the onerous process of creating a lab-grown SARS-CoV-2 strain that could be used to inoculate volunteers in the world’s first human challenge study for COVID-19.
“At the time, it was the height of the pandemic and with it being a new virus, nobody really knew how to properly grow it in a lab. We therefore had to do lots of sequencing to check the virus wasn’t changing as we propagated in the cultured cells”, she explains.
“We then had to work out how much virus to use because nobody knew when you caught COVID how many virus particles actually infected a person.
“The safety of our volunteers was paramount, so we decided to go in with an incredibly low dose, and we hit it spot on because half of the volunteers got infected and half of them didn't.
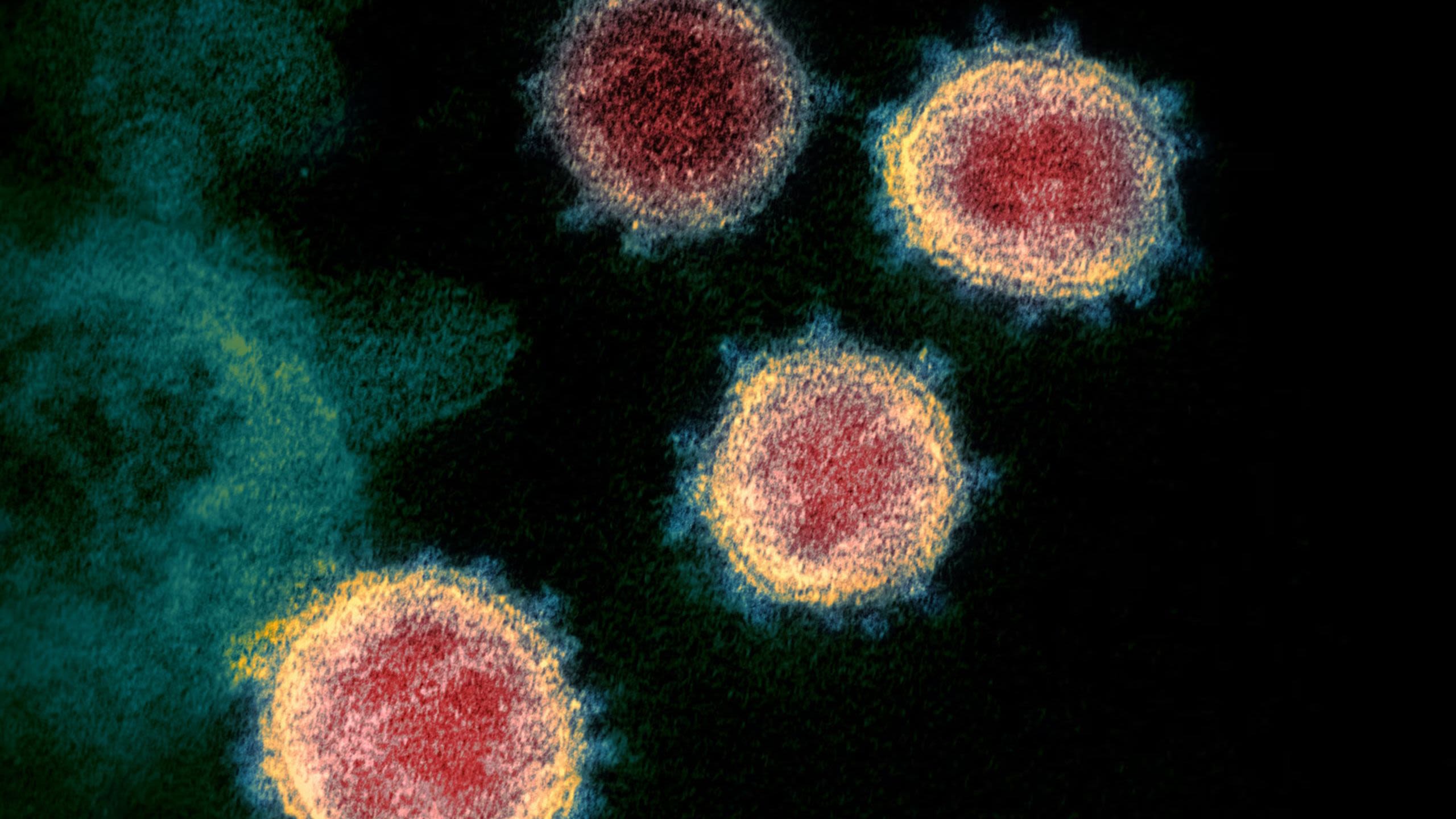
Microscope image of SARS-CoV-2, the virus that causes COVID-19
Microscope image of SARS-CoV-2, the virus that causes COVID-19
“In virology that's the perfect number because you get some people in whom you can study the virus growing intently, while from an immunology point of view, you've also got some people whose immune system is doing something for them to stop them getting infected, therefore allowing you to directly compare the groups.”
"The safety of our volunteers was paramount."
Dr Anika Singanayam, who served as Principal Investigator of the study, echoes Professor Barclay’s sentiments about the value of this type of study design: "We could take samples from the same person frequently and longitudinally, allowing us to understand things in a lot more detail. For example, when people were emitting the most virus in relation to their symptoms, and how much virus we could detect from a swab of their nose and throat. These sorts of things are difficult to look at through other study designs, or through natural infection studies."
Dr Anika Singanayam
Dr Anika Singanayam
One of the major insights gleaned from the research was that the nose is a significant route for infected people shedding virus into the air and environment. This highlighted the need to reinforce public health messaging around proper face mask use, hand washing and surface cleaning.
Looking ahead, Professor Barclay is keen to see human challenge studies move towards a ‘controlled transmission model’ for airborne viruses.
“To understand the transmission of a virus, you need two people, not one. So, what I’d like to do next is move towards a transmission model where you've got a ‘donor’ and a ‘sentinel’. The donor is the person who you know is infected and the sentinel is like the canary in the coal mine, who may or may not get infected depending on whether the donor is breathing out lots of infectious virus.
“Nobody's ever done this successfully in a human challenge model before, but I think that's what we need next. Because when the next outbreak comes, I honestly think that will be key to controlling an outbreak – understanding how you can stop a person transmitting to somebody else.”

The future of human challenge studies
As our researchers continue to innovate and pioneer new approaches in infection research, Imperial’s collaboration with clinical partners remains crucial.
In partnership with Chelsea and Westminster Hospital NHS Foundation Trust and its charity, CW+, Imperial is taking its next bold step: creating a dedicated human challenge facility in West London that draws on Imperial’s unique convening power.
By being co-located in a hospital, the new space will allow more detailed study of those taking part in studies and how infections transmit between individuals.
“One of the primary challenges in vaccine development is stopping the transmission of infections from one person to another. A facility that allows us to measure this will therefore be invaluable for advancing the creation of new vaccines for future respiratory viruses and tackling the growing threat of antimicrobial resistance,” explains Professor Graham Cooke, Vice Dean of Research in the Faculty of Medicine.
Professor Graham Cooke
Professor Graham Cooke
"Over the past 30 years, Chelsea and Westminster Hospital NHS Foundation Trust and Imperial College London have established a globally recognised partnership in infectious disease research. Together, we now have an opportunity to develop one of the most advanced human infection challenge centres in the UK, enabling us to carry out more studies and trials in a real-world clinical setting. This work will not only help us accelerate new treatment options for patients in our community, but could also resonate both nationally and internationally, helping us prepare for and respond quickly to future pandemics and create drug therapies that can halt the spread of infectious diseases," adds Roger Chinn, Chief Medical Officer at Chelsea and Westminster Hospital NHS Foundation Trust
Through initiatives like these, Imperial remains at the forefront, committed to sharing its expertise and driving global progress in the fight against infectious diseases.
“Our ambition has always been to innovate, pioneer and push the boundaries of science, as well as work with our partners locally and internationally to try to impact public health worldwide”, says Professor Cooke.
Supporters
Clinical research at Imperial College London is supported by funding from the NIHR Imperial Biomedical Research Centre (BRC), a translational research partnership between Imperial College London and Imperial College Healthcare NHS Trust, which was awarded £95m in 2022 to continue developing new experimental treatments and diagnostics for patients.
The MusICC consortium is supported by funding from the Coalition for Epidemic Preparedness Innovations (CEPI). Additional support for the SARS-CoV-2 controlled human infection programme comes from the Wellcome Trust, Bill and Melinda Gates Foundation, and HIC-Vac.
The CHMI-SIKA study is supported by funding from the Wellcome Trust, Alexander von Humboldt Foundation, The European & Developing Countries Clinical Trials Partnership (EDCTP), Initiative to Develop African Research Leaders (IDeAL), and Tackling Infections to Benefit Africa (TIBA) Partnership.
The CHANTS study is supported by funding from the Wellcome Trust, HIC-VaC, the BactiVac network, and the British Infection Association (BIA).